INTRODUCTION: Lymphodepleting chemotherapy (LDC) prior to CAR-T infusion is required for more effective expansion and persistence of infused T cells. Most commonly, fludarabine and cyclophosphamide (flu/cy) has been used. Given recent drug shortages, alternative chemotherapeutic agents such as bendamustine have been used, although limited data exists to evaluate its safety and efficacy. Our study aimed to investigate the outcomes and toxicity profile associated with the use of bendamustine compared to flu/cy LDC in patients receiving CD19 CAR T cells for B-cell non-Hodgkin lymphoma (B-NHL).
METHODS: In this retrospective analysis we included patients with relapse/ refractory B-NHL (follicular lymphoma (FL), mantle cell lymphoma (MCL), and large B cell lymphoma (LBCL)) treated with commercial CD19 CAR-T from Memorial Sloan Kettering Cancer Center (MSKCC) and Hackensack Meridian Health (HMH) between January 2020 to December 2022. We compared the outcomes and toxicity of those patients who received bendamustine LDC to historical controls treated with flu/cy. Response and toxicities including CRS and ICANS were assessed using the Lugano 2014 and ASTCT criteria, respectively.
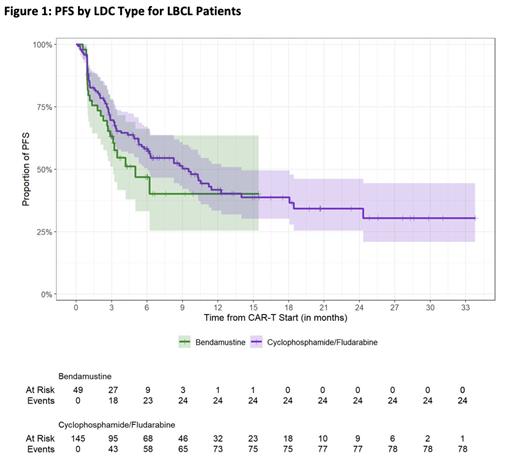
RESULTS: Two hundred and forty-two B-NHL (21 FL, 25 MCL, 196 LBCL) patients underwent CAR-T infusion, with 179 receiving flu/cy and 63 receiving bendamustine LDC. Baseline characteristics were comparable in each group except for number of lines of prior therapy, with the bendamustine group having received a median of 2 lines of prior therapy compared to 3 lines in the flu/cy group (p = 0.005). Patients with LBCL receiving bendamustine LDC had overall lower rates of all grade CRS (58% vs. 83%, p < 0.001), and all grade ICANS (6% vs. 31%, p < 0.001), as well as grade 3+ CRS (0% vs. 28%, p = 0.6) and grade 3+ ICANS (0% vs. 12%, p = 0.004). Additionally, LBCL patients receiving bendamustine had lower rates of D+30 neutropenia (absolute neutrophil counts 2.05 versus 1.40, p = 0.02), and a lower cumulative incidence of febrile neutropenia at D+30 (6.2% (95% CI 1.6 - 16) versus 38% (95% CI 30-46%)). The median follow-up for patients with LBCL who received bendamustine LDC was 4.3 months (95% CI 3.8 - 6) and 13 months (95% CI 12-17) in those receiving flu/cy LDC. Analysis of the best overall response rate (ORR) by day 100 of the LBCL cohort did not reveal any significant difference based on the type of LDC received (70% versus 77%, respectively, p = 0.4). Within the LBCL group, there was no significant difference in the bendamustine versus flu/cy groups with the estimated 1-year progression free survival at 40% (95% CI 25-64) versus 42% (95% CI 33-52) (Figure 1), and 1-year overall survival of 76% (95% CI 61-93) versus 68% (95% CI 59-77), respectively.
CONCLUSIONS: Bendamustine LDC appears to be a satisfactory LDC agent with a more favorable toxicity profile compared to flu/cy in patients receiving CD19 CAR T cells for B-NHL. While we noted comparable outcomes between the two groups, the short follow-up time in the bendamustine group currently limits a clearer assessment of potential differences in survival between the two groups. Longer follow up is necessary to ensure responses are sustained in those receiving bendamustine.
Disclosures
Ip:Genomic Testing Cooperative: Current equity holder in private company; Seattle Genetics: Speakers Bureau; AstraZeneca: Speakers Bureau; Secura Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MJH Life Sciences: Honoraria; COTA: Current holder of stock options in a privately-held company; Merck: Current holder of stock options in a privately-held company. Suh:Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Leslie:Eli Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; LRF: Other: Educational role; Celgene/ Bristol Myers Squibb: Other: Travel support, Speakers Bureau; SeaGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; CLL: Other: Educational role; Astrazeneca: Consultancy, Other: Travel support, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen/ J&J: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; LLS: Other: Educational role/ Leadership role in LLS light the night events (unpaid); Janssen/PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Giralt:Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding; Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees. Palomba:Synthekine: Honoraria; Kite: Honoraria; Ceramedix: Honoraria; Juno: Honoraria, Patents & Royalties; Thymofox: Honoraria; Pluto Immunotherapeutics: Honoraria; MustangBio: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; GarudaTherapeutics: Honoraria; Rheos: Honoraria; Novartis: Honoraria; Smart Immune: Honoraria; Cellectar: Honoraria; BMS: Honoraria. Salles:Orna: Consultancy; Ipsen: Consultancy, Research Funding; Owkin: Current holder of stock options in a privately-held company; Loxo/Lilly: Consultancy; Nordic Nanovector: Consultancy; ATB Therapeutics: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy, Honoraria; BMS/Celgene: Consultancy; Debiopharm: Consultancy; Kite/Gilead: Consultancy; Janssen: Consultancy, Research Funding; Incyte: Consultancy; Genmab: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; EPIZYME: Consultancy; Merck: Consultancy, Honoraria; Molecular Partners: Consultancy; Novartis: Consultancy; Nurix: Consultancy. Shah:ArcellX: Other: DSMB; Janssen: Research Funding; BMS: Research Funding; Beyond Spring: Research Funding; Amgen: Research Funding. Perales:NexImmune: Consultancy, Current equity holder in publicly-traded company; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Kite: Consultancy, Honoraria, Research Funding; Caribou: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Allogene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Exevir: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; DSMB: Other; BMS: Consultancy, Honoraria; Sellas Life Sciences: Consultancy; Medigene: Consultancy, Other; Cidara Therapeutics: Consultancy, Other; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Miltenyi Biotec: Honoraria; Allovir: Consultancy; Astellas: Consultancy, Honoraria; Syncopation: Honoraria; Celgene: Honoraria; Takeda: Consultancy, Honoraria; Adicet: Honoraria; Vor Biopharma: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Servier: Other. Scordo:Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; CancertNetwork (Intellisphere LLC): Honoraria; Medscape, LLC: Honoraria; Angiocrine Bioscience, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal